In part 1 I covered issues related to the effect of TRT (Testosterone Replacement Therapy) on male fertility. Here I will outline options for men to increase endogenous testosterone production by non-TRT means, and ways to speed up spermatogenesis for those who chose to go the TRT route…
If you missed part 1 on this topic, here it is http://www.brinkzone.com/mens-health/testosterone-replacement-therapy-trt-and-fertility-how-to-get-the-best-of-both-worlds-part-1
The same strategies apply to increasing endogenous testosterone production and speeding up its recovery after supplementation, as illustrated in the figure below (click image to see it in full).
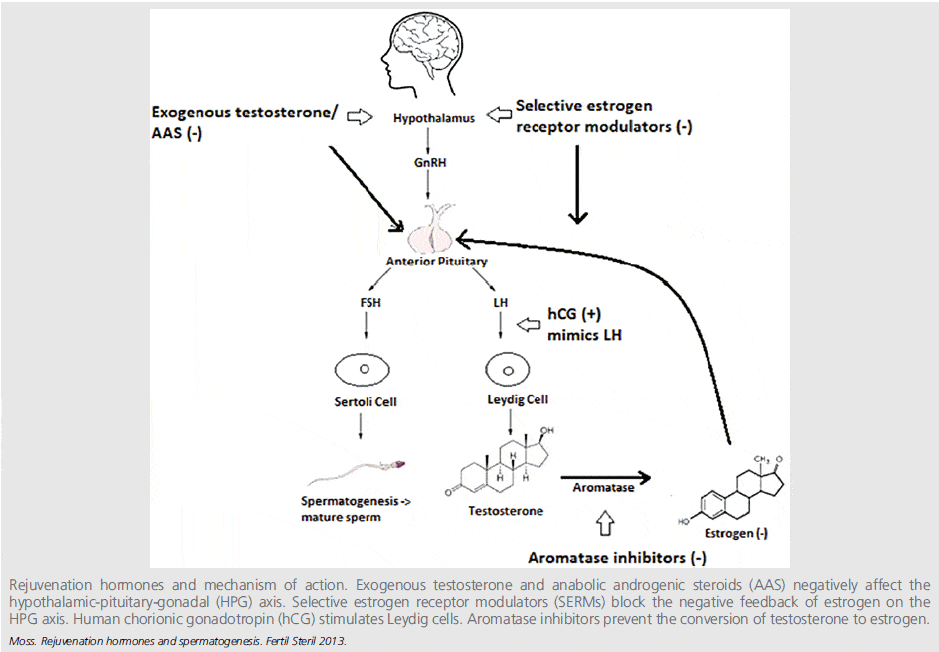
HCG (Human Chorionic Gonadotropin)
HCG is an LH analogue and thus stimulates testicular (Leydig cell) production of testosterone, and increases both intra-testicular testosterone (ITT) concentrations and serum T levels.
A study induced experimental gonadotropin deficiency in 37 normal men with gonadotropin-releasing hormone (GnRH) antagonists and randomized the men to receive one of four doses of HCG: 0, 15, 60, or 125 IU subcutanesouly every other day for 10 days.[1] Testicular fluid was obtained by percutaneous aspiration for steroid measurements at baseline and after 10 days of treatment. The ITT levels increased in a dose-dependent manner, with very low-dose HCG administration Moreover, blood levels of HCG were significantly correlated with both ITT and blood testosterone levels. It was concluded that doses of HCG that ware far lower than those typically used clinically (1,000–2,000 IU SC three times weekly) increased ITT concentrations in a dose-dependent manner in normal men with experimental gonadotropin deficiency.[1]
To investigate how HCG affects the different fractions of serum T, eugonadal healthy men were injected with 6000 IU HCG i.m.[2] Blood was collected every 10 min for 2.5 h and then once a day for 4 days. By day 4 the mean serum testosterone level had risen to 178% +/- 13% (SEM) of a mean basal level (first five samples). This rise was less than that in free testosterone (221% +/- 18%), which was in turn less than the rise in the non-SHBG-bound fraction (255% +/- 19%). The concentrations of SHBG and albumin were constant. Thus, in healthy men HCG increases the bioactive fraction of testosterone (free and non-SHBG-bound) to a greater extent than the TT level. Thus, when on an HCG cycle it is important to do blood work and measure all T fractions in order to monitor treatment efficacy.
Most clinical research has studied HCG treatment on patients with hypogonadism induced by pituitary disorders, idiopathic or genetic abnormalities. Therefore it might not be directly applicable to fertility issues related to TTh. A better model for TTh related infertility might be to study HCG intervention outcomes after anabolic steroid use in men without background pituitary or testicular abnormalities. In common with anabolic steroid use, TTh exogenously elevates testosterone levels and suppress LH and FSH, and thereby can cause infertility.
To put the data on HCG in perspective it is interesting to look at some case study. A married couple with primary sub-fertility secondary to anabolic steroid-induced azoospermia that was persistent despite 1 year of cessation from steroid use.[3] The husband was a bodybuilder who admitted to have used the anabolic steroids testosterone cypionate, methandrostenolone, oxandrolone, testosterone propionate, oxymetholone, nandrolone decanoate, and methenolone enanthate. The patient received twice-weekly injections of 10,000 IU of HCG and daily injections of 75 IU of HMG (menopausal gonadotropins) for 3 months. Semen analyses returned to normal after 3 months of treatment. The couple conceived spontaneously 7 months later. Thus, anabolic steroid-induced azoospermia which is persistent after cessation anabolic steroid use can be treated successfully with HCG and HMG. Another Study also shows that IM injections of HCG 2-3 times per week at doses of 2,000–3,000 IU for 4 months can initiate spermatogenesis after anabolic steroid-induced azoospermia.[4]
HCG + T
HCG not only helps resume spermatogenesis after a TRT cycle, but also can be used in conjunction with TRT to preserve sperm quality. In a study men with normal reproductive physiology were randomized to receive 200 mg T enanthate weekly in combination with either saline placebo or 125, 250, or 500 IU hCG every other day for 3 wk. ITT was assessed in testicular fluid obtained by percutaneous fine needle aspiration at baseline and at the end of treatment. [5] Baseline serum T (409 ng/dl, 14.1 nmol/liter) was 1.2% of ITT (34,046 ng/dl, 1174 nmol/liter). In the T enanthate/placebo group, LH and FSH were profoundly suppressed to 5% and 3% of baseline, respectively, and ITT was suppressed by 94%. In the T enanthate/HCG groups, ITT increased linearly with increasing hCG dose. Post-treatment ITT was 25% less than baseline in the 125 IU hCG group, 7% less than baseline in the 250 IU hCG group, and 26% greater than baseline in the 500 IU hCG group. These results demonstrate that relatively low dose hCG maintains ITT within the normal range in healthy men with gonadotropin suppression due to T supplementation. [5]
Another study confirmed that intra-testicular testosterone can be maintained during testosterone replacement therapy with co-administration of low dose HCG, which may support continued spermatogenesis in patients on testosterone replacement therapy.[6] 26 hypogonadal men with a mean age of 35.9 years were included. TRT was combined with intramuscular injections of human chorionic gonadotropin (500 IU) every other day. Mean follow-up was 6.2 months. Of the men 19 were treated with injectable testosterone and 7 were treated with transdermal gel. Mean serum hormone levels before vs during treatment were testosterone 207.2 vs 1,055.5 ng/dl, free testosterone 8.1 vs 20.4 pg/ml and estradiol 2.2 vs 3.7 pg/ml. Pretreatment semen parameters were volume 2.9 ml, density 35.2 million per ml, motility 49.0% and forward progression 2.3. No differences in semen parameters were observed during greater than 1 year of followup. No impact on semen parameters was observed as a function of testosterone formulation. No patient became azoospermic during concomitant testosterone replacement and human chorionic gonadotropin therapy. Nine of 26 men contributed to pregnancy with the partner during followup. It was concluded that HCG appears to maintain semen parameters in hypogonadal men on testosterone replacement therapy. Concurrent testosterone replacement and human chorionic gonadotropin use may preserve fertility in hypogonadal males who desire fertility preservation while on testosterone replacement therapy.
Clomiphene citrate (CC)
CC works by blocking estrogen (E) action in the pituitary.[7] The pituitary thereby sees less estrogen and makes more LH due to removal of negative feedback inhibition. Increased LH levels in turn stimulate the Leydig cells in the testis to synthesize more testosterone.
Oral CC has been shown, in doses of 25 mg per day or 50 mg every other day for up to 19 months, to be safe and effective in the treatment of testosterone deficiency (aka hypogonadims) and infertility in men.[8-14] At a dose of 100 mg/day for 2 months, clomid has also been shown to successfully reverse testosterone deficiency symptoms, restore testosterone levels, LH surges and the hypothlamic-pituitary-gonadal axis in a 30 year old male patient whose axis was previously shut off with multiple anabolic steroid abuse.[15]
An interesting and practically relevant study was conducted to compare the effectiveness of clomid versus testosterone gel replacement therapy on biochemical and clinical efficacy, and treatment cost:[16]
Main Outcome Measures
The main outcome measures were change in serum testosterone with CC and TGRT therapy, and change in the androgen deficiency in aging male (ADAM) questionnaire scores with CC therapy.
Methods:
104 men with hypogonadism (defined as testosterone below 300 ng/mL) or infertility, were divided into two groups:
Clomid group – average age 42 years, receiving 50 mg clomid every other day.
Testosterone Gel group – average age 57 years, receiving 5 g/day of either Androgel® 1% or Testim® 1%.
Blood values were collected 1–2 months after treatment initiation and semi-annually thereafter. Retrospective data collection was performed via chart review.
Subjective follow up of patients receiving clomid was performed via telephone interview using the ADAM questionnaire.
Results:
Average follow up was 23 months (Clomid group; range 8–40 months) vs. 46 months (Testosterone Gel group; range 6–149 months).
The treatments significantly increased total testosterone levels to 573 ng/dL in the Clomid group (baseline, 277 ng/dL) and 553 ng/dL (baseline, 221 ng/dL) in the Testosterone Gel group.
The monthly treatment cost, as per 2009, was:
– Testim® 1% (5 g daily) = $270
– Androgel® 1% (5 gm daily) = $265
– Clomid (50 mg every other day) = $83
Symptom scores improved significantly among clomid treated patients; the average pre-treatment ADAM score was 4.9 vs. 2.1 at follow up.
Average pretreatment ADAM sexual function domain score was 0.76 vs. 0.23 at follow up. There were no adverse events reported.
Study Conclusion
Clomid (clomiphene citrate) is a good treatment option for men with hypogonadism, demonstrating biochemical and clinical efficacy with few side effects and much lower cost compared with testosterone gel.
While clomid may be an effective option to treat testosterone deficiency and infertility in men, it is important to note that it does reduce GH ( growth hormone) secretion [17], lowers IGF-1 levels[18], and elevates SHBG levels (thereby lowering active free testosterone levels).[18] Therefore, during clomid treatment it is important to monitor the GH/IGF-1 axis and levels of SHBG and free testosterone, in addition to total testosterone levels and symptoms. It should also be noted that the efficacy of CC treatment in raising T levels and improving symptoms might be reduced in older men over 65 years of age (due to impairment of testicular function) [10] and in men with diabetes, hypertension, coronary artery disease, and multiple medication use.[8]
Bottom Line
The good news is that there are effective options for men who are concerned about their fertility. Both HCG, HCG+T and clomid have good research backing up their efficacy.
When using HCG or clomid, it is important to do regular blood work and check all T fractions in order to monitor treatment efficacy. Also, when using clomid it is especially important to monitor IGF-1 levels as well.
Clomid mono-therapy is an effective way to increase T levels in the physiological range, and is cheaper that T gel. Clomid is also easier to administer as it is an oral, and not an injectable like HCG.
The studies outlined in this article give directions on dosages and treatment duration. However, it is important to remember that there are great inter-individual differences in endocrinology, especially when it relates to the hypothalamus, pituitary and testis.[19-21] Therefore it is critical to go to a doctor who is well versed in the area, and who regularly have patients do blood work.
The importance of blood work cannot be overstated.
References:
1. Roth, M.Y., et al., Dose-dependent increase in intratesticular testosterone by very low-dose human chorionic gonadotropin in normal men with experimental gonadotropin deficiency. J Clin Endocrinol Metab, 2010. 95(8): p. 3806-13.
2. Cooke, R.R., et al., Serum forms of testosterone in men after an hCG stimulation: relative increase in non-protein bound forms. Clin Endocrinol (Oxf), 1990. 32(2): p. 165-75.
3. Menon, D.K., Successful treatment of anabolic steroid-induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin. Fertil Steril, 2003. 79 Suppl 3: p. 1659-61.
4. Turek, P.J., et al., The reversibility of anabolic steroid-induced azoospermia. J Urol, 1995. 153(5): p. 1628-30.
5. Coviello, A.D., et al., Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab, 2005. 90(5): p. 2595-602.
6. Hsieh, T.C., et al., Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol, 2013. 189(2): p. 647-50.
7. Kim, E.D., et al., The treatment of hypogonadism in men of reproductive age. Fertil Steril, 2013. 99(3): p. 718-24.
8. Guay, A.T., et al., Clomiphene increases free testosterone levels in men with both secondary hypogonadism and erectile dysfunction: who does and does not benefit? Int J Impot Res, 2003. 15(3): p. 156-65.
9. Shabsigh, A., et al., Clomiphene citrate effects on testosterone/estrogen ratio in male hypogonadism. J Sex Med, 2005. 2(5): p. 716-21.
10. Tenover, J.S. and W.J. Bremner, The effects of normal aging on the response of the pituitary-gonadal axis to chronic clomiphene administration in men. J Androl, 1991. 12(4): p. 258-63.
11. Guay, A.T., S. Bansal, and G.J. Heatley, Effect of raising endogenous testosterone levels in impotent men with secondary hypogonadism: double blind placebo-controlled trial with clomiphene citrate. J Clin Endocrinol Metab, 1995. 80(12): p. 3546-52.
12. Moskovic, D.J., et al., Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int, 2012. 110(10): p. 1524-8.
13. Katz, D.J., et al., Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int, 2012. 110(4): p. 573-8.
14. Da Ros, C.T. and M.A. Averbeck, Twenty-five milligrams of clomiphene citrate presents positive effect on treatment of male testosterone deficiency – a prospective study. Int Braz J Urol, 2012. 38(4): p. 512-8.
15. Tan, R.S. and D. Vasudevan, Use of clomiphene citrate to reverse premature andropause secondary to steroid abuse. Fertil Steril, 2003. 79(1): p. 203-5.
16. Taylor, F. and L. Levine, Clomiphene citrate and testosterone gel replacement therapy for male hypogonadism: efficacy and treatment cost. J Sex Med, 2010. 7(1 Pt 1): p. 269-76.
17. Perlow, M., et al., Reduction of growth hormone secretion following clomiphene administration. Metabolism, 1973. 22(10): p. 1269-75.
18. Butzow, T.L., L.M. Kettel, and S.S. Yen, Clomiphene citrate reduces serum insulin-like growth factor I and increases sex hormone-binding globulin levels in women with polycystic ovary syndrome. Fertil Steril, 1995. 63(6): p. 1200-3.
19. Crabbe, P., et al., Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: contribution of the androgen receptor polyglutamine tract polymorphism. J Clin Endocrinol Metab, 2007. 92(9): p. 3604-10.
20. Carruthers, M., The paradox dividing testosterone deficiency symptoms and androgen assays: a closer look at the cellular and molecular mechanisms of androgen action. J Sex Med, 2008. 5(4): p. 998-1012.
21. Tirabassi, G., et al., Androgen Receptor Gene CAG Repeat Polymorphism Regulates the Metabolic Effects of Testosterone Replacement Therapy in Male Postsurgical Hypogonadotropic Hypogonadism. Int J Endocrinol, 2013. 2013: p. 816740.




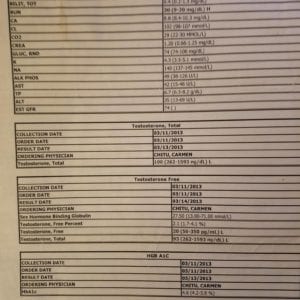

Hi Monica, I am 56 and live in the UK. My GP recently sent me for blood works and amongst the tests was a serum T level – the score that came back was 16.9 but while my GP said that was in a “normal” range, I have no idea what it means as I can’t see any similar types of scores in your articles. Please help. Thanks, Gary
Find out what unit: ng/mL or pg/mL
The unit of the measure tells if it is free or total T.
Excellent work Monica!
Thank you Jim. 🙂
Great article. I am just amazed that the Doctors that are accessible to us on the regular insurance plans and specialize in this area are so clueless. They should be trying to access this type of information and educating themselves more thoroughly in this area.
There are several reasons for this, including (but not limited to):
1) old-school dogma mindset
2) lack of interest
3) lack of time to stay on top recent research findings
Greetings from Australia, to Will and Monica.
I must say, it is a rarity to be able to absorb an article and when finished, immediately feel that you’ve been educated. Not run around.
Impeccably presented, Monica. look forward to reading more of your articles.
Hey Will, great job done in choosing someone of this calibre to present information in their field.
Just loved this, and will recommend this site to my friend who also trains, and is interested in the ‘real’ advanced knowledge on the subject.
Thanks for what you have shared with us,
Bruce
Thank you very much Bruce. 🙂
Hi Monica, I really like your analytical approach but I feel it’s an uphill battle when seemingly qualified people like Dr. Neil Goodman (an endocrinologist) write articles like this on on his perspective of TRT http://www.cagepotato.com/heres-what-happens-to-your-body-when-you-stop-using-trt/
What are your thoughts on his perspective?
Regards, Gary
That article covers the issue of TRT in competitive athletes, which is not applicable to the main part of the population.
It has to be remembered that there is not one single T level that it optimal for everybody due to differences in AR (androgen receptor) sensitivity.. Many factors need to be taken into consideration, eg. blood lipids, lipoproteins, glucose, fat mass, libido, psychological symptoms etc etc.
Both too low and too high T levels (like any other physiological parameter) are deleterious. The key is for everybody to find what’s their individual optimal point.
Interesting article. Could a GnRH stimulant be better suited to prevetn Azoospermia during TRT?
I have read your blog, it was very informative. I was looking for such a blog from a long time. It has given me appropriate knowledge about fertility and pregnancy. Just through your blog, I came to know about Ovulation calculator. Now it will be easy for me to determine my fertility days, as I am planning to get pregnant. Due my husband’s job, he is mostly out of station and it becomes difficult for us to coordinate during my fertility day. Now we will co-opt, so that I can get pregnant.